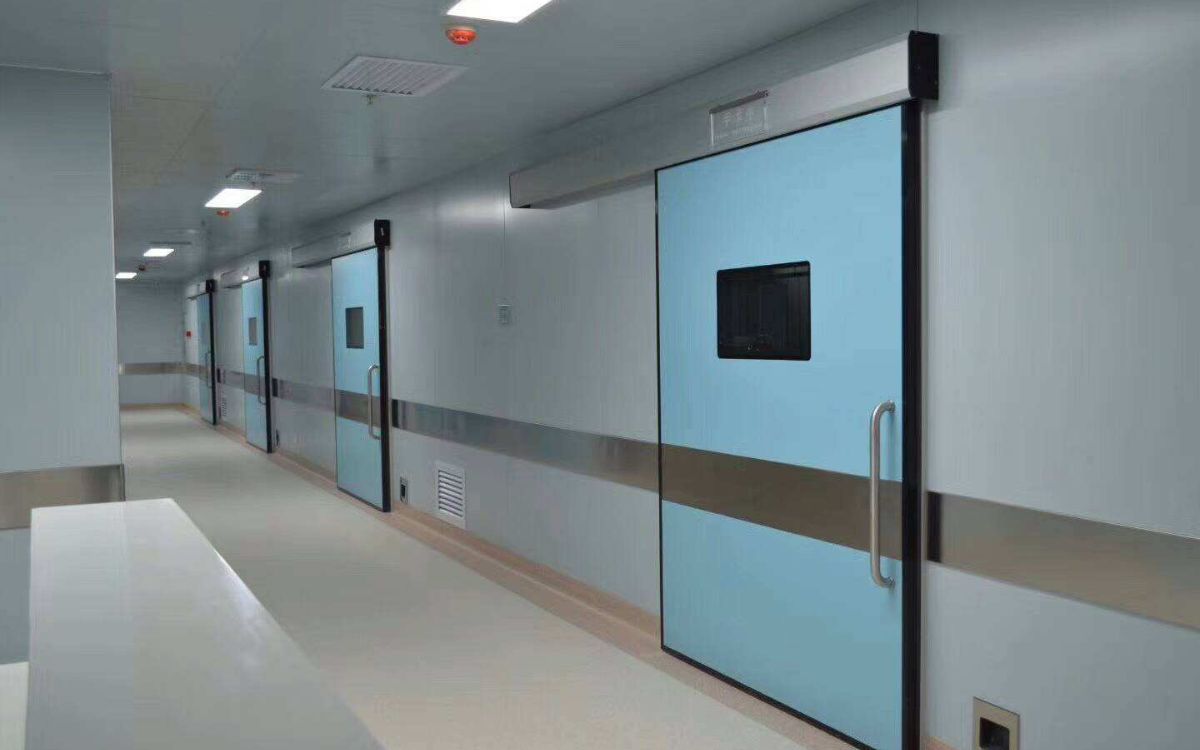
Automatic doors help control contamination, maintain pressure and reduce hand contact in cleanrooms. The article “When to invest in automatic doors in cleanrooms” will point out the cases where they should be applied such as ISO 5–7 level areas, high traffic or one-way process requirements.
- 1. Factors Influencing the Decision to Invest in Automatic Doors
- 2. Scenarios Where Automatic Doors Should Be Considered
- 3. Long-term Benefits of Automatic Doors in Cleanroom Environments
- 4. Comparison: Manual vs. Automatic Doors - When to Choose Which?
- 5. Which Industries Should Prioritize Automatic Doors?
- 6. Quick Answers
- 7. Unsure Which Areas Should Use Automatic Doors?
1. Factors Influencing the Decision to Invest in Automatic Doors
Contamination Control Level (Cleanroom Class)
Automatic doors are often prioritized in high-grade cleanrooms (ISO 5-ISO 7 or GMP Grade A/B), where maintaining pressure differentials, particle limits, and minimizing cross-contamination are mandatory. Automated door operation reduces the risk of airborne contaminants entering due to manual door handling, especially in high-traffic areas.
Traffic Flow of Personnel and Materials
In areas with heavy foot traffic—such as during shift changes or frequent material handling—manual doors can lead to:
- Congestion at doorways
- Increased physical contact → hygiene risk
- Doors left open unintentionally → pressure loss
Automatic doors optimize workflow, save time, and reduce operational risks.
One-Way Workflow and Contact Limitation Requirements
In GMP or ISO-compliant facilities, one-way movement is essential to prevent cross-contamination. Automatic doors support this model effectively by:
- Configurable one-way opening
- Integration with motion or RFID sensors to reduce hand contact
- Enabling movement tracking and flow control

Integration with Interlock and Access Control Systems
Many modern facilities implement interlock or access control systems. Within these systems:
- Manual doors often compromise process accuracy
- Errors in timing or operation (e.g., simultaneous opening) can occur
Properly integrated automatic doors enhance system synchronization, process stability, and overall intelligent facility management.
See more: Why Automatic Doors are the Superior Choice for Pharmaceutical Cleanrooms
2. Scenarios Where Automatic Doors Should Be Considered
Not every area within a cleanroom requires automatic doors. However, in key functional zones—where manual operation could interrupt workflow or raise contamination risks—automatic doors are highly recommended. Below are typical use cases:
|
Area |
Why Automatic Doors are Recommended |
|
Weighing Room |
This is a sensitive zone where powder particles or contaminants from active ingredients may be released. Automatic doors minimize hand contact and reduce cross-contamination—especially in rooms requiring double protection (e.g., glove boxes + airlocks). |
|
Production Area Airlocks |
As a transition zone between cleanroom classes, airlocks benefit from automatic doors to maintain pressure stability and prevent simultaneous door openings. They also regulate personnel flow and minimize manual operation errors. |
|
Pass-through Zones |
When combined with automatic or semi-automatic pass boxes, automatic doors streamline material transfers and ensure proper door sequencing, avoiding operational errors and excessive door opening times. |
|
Packaging and Filling Rooms |
In continuous production zones, automatic doors can be synchronized with production lines to prevent interruptions and maintain clean air standards. |
Tip: Consider integrating motion sensors, RFID, or touchless switches to improve operational efficiency and hygiene in these zones.
See more: Choose the right Cleanroom Door with 5 factors
3. Long-term Benefits of Automatic Doors in Cleanroom Environments
Investing in automatic doors delivers more than just convenience—it enhances operational consistency, regulatory compliance, and long-term cost-efficiency in controlled environments.
Improved Hygiene and No-touch Operation
In environments requiring absolute sterility or minimal contact (e.g., pharmaceuticals, cosmetics, nutraceuticals), avoiding surface contact is crucial. Automatic doors with sensors or touchless activation:
- Eliminate manual door handling
- Prevent cross-contamination between clean and non-clean zones
- Comply with GMP/ISO hygiene requirements
Reduced Operational Errors from Unclosed Doors
A common issue in cleanrooms is failure to close doors, especially during busy operations or multitasking. This can result in:
- Pressure imbalance between rooms
- Infiltration of dust or microbes
- Breach of one-way flow requirements, affecting GMP audits
Automatic doors close immediately after passage, with controlled delay settings—eliminating such manual errors.

Enhanced Process Control (with Access Control Integration)
When integrated with access control or interlock systems, automatic doors contribute to intelligent operations by:
- Restricting access to authorized personnel only
- Logging entry and exit records by location
- Enforcing sequential operations (e.g., one door opens only after another is fully closed)
This is especially effective in pharmaceutical manufacturing, R&D labs, or data-sensitive clean zones.
Long-term Operational Cost Optimization
While initial costs are higher than manual doors, automatic systems offer better return on investment:
- Lower maintenance (no slamming, reduced wear)
- Longer lifespan due to controlled operation
- Reduced labor for door supervision
- Fewer contamination-related incidents and corrective costs
Total Cost of Ownership (TCO) is lower over 5-10 years, especially in large-scale GMP-compliant facilities.
4. Comparison: Manual vs. Automatic Doors - When to Choose Which?
Choosing between manual and automatic doors is not solely a matter of budget. It also depends on the door’s function, installation location, environmental control requirements, and operational flow. The comparison table below helps determine the appropriate type of door for each cleanroom zone:
|
Criteria |
Manual Door |
Automatic Door |
|
Investment Cost |
Low - suitable for limited budgets or areas with minimal control requirements |
Higher - requires motor, sensor, and controller installation |
|
Biosafety Level |
Medium - prone to hand contact, depends on manual handling |
High - touch-free, reduces cross-contamination, supports strict process control |
|
User Convenience |
Manual operation - requires physical effort, tiring with high usage |
Automatic operation - opens via sensor or touchless button, integrates with access control |
|
Recommended Areas |
- Secondary changing rooms - Technical hallways - Support storage zones |
- Airlocks - Weighing rooms - GMP-grade packaging and filling areas - Cleanroom main access points |
When to Choose Manual Doors:
- Projects with limited budgets or temporary retrofits
- Areas without high cleanroom requirements
- Zones with low traffic and minimal contamination risk
When to Choose Automatic Doors:
- Critical control zones: GMP or ISO 7+
- High-traffic areas for personnel or materials
- Closed-loop, non-contact workflow requirements
- Integration with interlock or access control systems
See more: Latest price list of clean room doors used in cosmetic factories
5. Which Industries Should Prioritize Automatic Doors?
Not all industries require automatic doors in their cleanroom setups. However, in sectors with strict contamination control, continuous operations, and compliance with international standards, automatic doors are a highly recommended long-term investment.
Pharmaceuticals - GMP-Mandated in Critical Areas
In EU-GMP or WHO-GMP compliant pharmaceutical plants, controlling cross-contamination and personnel flow is critical. Automatic doors are considered standard in:
- Weighing rooms: Minimize airborne dust, protect staff and test materials
- Filling and bottling rooms: Ensure no contact, prevent microbial contamination
- Main airlocks to Grade A/B zones: Maintain pressure differential and clean airflow

Food - Ensuring Hygiene at Transition Points
The food industry often demands tight control over dust, bacteria, and insect ingress, especially at high-risk transitions:
- End-of-line packaging zones: Rapid operation needed, frequent use → sensor-based doors save time
- Cold storage and preparation rooms: Manual doors may not seal completely, leading to energy loss and product spoilage
Cosmetics - Process Hygiene and Controlled Entry
In cosmetics manufacturing, particularly for natural or handmade products, hygiene and procedural control are essential for brand trust:
- Changing rooms (post-handwashing): Automatic doors reduce surface contact
- Internal airlocks: Enforce one-way movement, prevent simultaneous opening of multiple doors
Electronics - ESD and Precision Control
In electronics, semiconductors, or SMT production, maintaining ISO 5-6 cleanroom standards and controlling electrostatic discharge (ESD) is critical:
- Automatic doors offer stable, contact-free operation, minimizing static buildup
- Can be integrated with ESD sensors and user authentication systems for smart workflows
See more: Supplier of clean room doors meeting GMP standards according to ISO 14644
6. Quick Answers
Is it mandatory to use automatic doors in GMP-compliant facilities?
Not for the entire facility, but they are required in critical areas such as weighing rooms, primary airlocks, and Grade A/B zones—where contamination prevention and unidirectional flow are strictly enforced.
Can automatic doors be integrated with an interlock system?
Yes. In fact, integration is recommended to ensure only one door opens at a time. Combining with interlock systems helps maintain pressure differentials, prevent procedural errors, and meet transition zone control standards.
Can a manual door be converted into an automatic door?
Yes, but several considerations must be addressed:
- Evaluate the door leaf and frame for mechanical durability
- Check electrical access, power supply, and motor space
- Recalculate the position for sensors, control buttons, or navigation systems
7. Unsure Which Areas Should Use Automatic Doors?
Choosing the right door type doesn’t just affect upfront costs—it determines operational efficiency and long-term compliance with GMP/ISO standards.
You can send your cleanroom layout or facility diagram to the VCR technical team. We will help you:
- Identify zones where automatic doors are needed
- Recommend door types based on cleanroom classification
- Advise on system integration with interlock, sensors, and access control
Free Technical Consultation - Preliminary Design Based on Industry Standards
Hotline: 090.123.9008
Email: [email protected]
Website: https://cuaphongsachvcr.com/
Diep VCR